| Issue |
Europhysics News
Volume 56, Number 3, 2025
Soft matter physics
|
|
|---|---|---|
| Page(s) | 17 - 20 | |
| Section | Features | |
| DOI | https://doi.org/10.1051/epn/2025308 | |
| Published online | 31 July 2025 | |
Coacervates are the soft matter of life
Institute for Molecules and Materials, Radboud University, Coacervates and Soft interfaces group Institute for Molecules and Materials 6525 AJ Nijmegen The Netherlands
The discovery that coacervates – dense aqueous droplets formed by phase separation – play a role in almost every process taking place in our cells leads to new perspectives on soft matter in living systems. Studying the soft material properties and chemical capabilities of coacervates is opening new avenues in pharmacology and biomaterials.
© European Physical Society, EDP Sciences, 2025
Life is remarkable. Each cell in an organism is composed of an enormous complexity of molecules, many of them large proteins and nucleic acids. Together they function in a controlled and coordinated manner, as if each molecule knew where it had to go and when. Building the same cell by mixing together the different proteins would inevitably result in a lifeless chunk. Instead, cellular components need to be carefully organized into dynamic compartments, as many proteins interact with diverse partners, require different environments, and are activated at different locations and times. Think about how we store different types of food together in boxes, bottles and bags, either in cupboards or the fridge. You take out the right ingredients when you are cooking, and their combination brings out the right flavours.
Numerous studies in the past decade have revealed that phase separation plays a key role in the formation of these dynamic compartments in cells, most of which are smaller than a micrometre. Physically, weak multivalent interactions between interacting molecules drives their phase separation into droplets, which are called condensates or coacervates (Figure 1) [1]. Unlike the textbook examples of membrane-bound compartments in the cell, such as the nucleus, Golgi and mitochondria, these coacervate-based compartments have no membrane. On the one hand, this makes them highly dynamic, able to freely take in other biomolecules, and amenable to formation and dissolution on demand. On the other hand, it also makes them unstable, prone to ripening and fusion. How cells manage to deal with the limited stability of coacervates is a topic of active research. Presumably, they use a combination of classical physicochemical strategies – surfactants and Pickering stabilization – and active droplet turnover with constant energy dissipation to control the size of the intracellular emulsion formed by coacervate droplets [2]. At the same time, by taking advantage of the natural coarsening of emulsions, coacervates can also be made much larger. Coacervate droplets of tens of micrometres have been used as mimics of entire cells with comparable crowding and softness. Beyond that, bulk coacervate-based materials are used as bioadhesives or in wound healing.
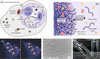 |
Fig. 1: Coacervates as phase-separated droplets in biology: (a) A mammalian cell with various types of membraneless organelles, (b) free exchange of proteins and small molecules in one such coacervate-based compartment due to a lack of membrane, (c) example of liquid nature of phase-separated droplets of small heat shock protein B2 (HspB2) in the nucleus of HEK293 cells, (d) synthetic coacervate droplets made from short peptide derivatives, (e) upon fusion and settling, coacervates can form macroscopic dense liquid phases. The position of the interface is highlighted with an arrow. |
Therefore, attention has now also been directed at the physical properties of coacervates: they represent a unique class of soft matter, tailored to be responsive to forces in cells and interacting with materials found in nature. Here, we describe how the phase-separated nature of coacervates gives rise to one-of-a-kind soft matter properties, ranging from transformation into fibrous solids, adhesion to soft, wet and slippery surfaces, and the ability to exert forces through capillarity. These properties open new avenues in pharmacology and biomaterials.
Opposites attract
Coacervates form through liquid-liquid phase separation in aqueous solutions. Entropically, mixing is always favoured, but demixing can occur if the interactions between molecules are strong enough. Crucial for the formation of coacervates is that the interacting molecules remain in a liquid condensed state, which is strongly hydrated. This requires flexible (e.g., polymeric) molecules, and weak, multivalent interactions. Coacervates are usually formed by polymers that exhibit attractive interactions, such as between opposite charges, aromatic groups or cations and aromatic groups. If these interactions occur between different molecules, phase separation results in so-called complex coacervates that contain both molecules in a stoichiometric ratio. If these interactions occur between different parts in the same molecules, phase separation results in so-called simple coacervates that contain a single type of molecule.
Repulsive interactions can also give rise to liquid-liquid phase separation: in this case, two water-soluble polymers with mutually unfavourable interactions will separate in two aqueous phases, each enriched in one of the polymers. This “segregative” phase separation is usually called an aqueous two-phase system. Despite the vast diversity in chemical interactions, classical polymer physics frameworks, including Flory-Huggins theory and percolation-coupled-to-phase-separation, have been successful at describing the phase behaviour of coacervates and aqueous two-phase systems almost quantitatively [3]. Both from a chemical and soft matter perspective, the difference between simple coacervate, complex coacervates and aqueous two-phase systems is also not of great importance: droplets formed in all cases can take up and concentrate other solutes, they are all viscoelastic liquids or glassy solids, they can stick to surfaces and exert capillary forces.
In the cell, the weak multivalent interactions required for coacervation are ubiquitous: intrinsically disordered proteins and nucleic acids contain many exposed charged, aromatic and hydrophobic residues ready to interact. Similarly, synthetic coacervates are also typically made with oppositely charged polymers, although there is an increasing number of examples of small molecules, short peptides and amphiphiles that are also capable of forming coacervates.
Soft coacervates
While the term liquid-liquid phase separation suggests that coacervates are simple liquids, the polymeric nature of many coacervate components and the high concentrations reached in the coacervate phase, make the material properties of coacervates very interesting. Directly after formation, most coacervates are best described as Maxwell fluids, with typical relaxation timescales of seconds to minutes, and crossover moduli of 1 Pa to 1 kPa, firmly in the regime of soft matter [4]. Indeed, coacervates can easily be deformed and manipulated by collective action of motor proteins, filaments and enzymes such as RNA polymerase [5].
The weak interactions that collectively give rise to a network fluid with these soft material properties also underlie the low interfacial tension of coacervates, which is typically between 1 and 100 μN/m – a thousand times lower than the interfacial tension between oil and water. The fluid nature distinguishes coacervates from hydrogels. Coacervates spread on many interfaces, including membranes, and although the interfacial tension is low, coacervates can strongly deform membranes and even induce membrane scission [6] Similarly, coacervate capillary forces are sufficiently strong to reposition genomic loci in the nucleus within seconds and manipulate chromatin structure [7]. It seems that the weak molecular interactions underlying coacervate formation also make coacervates ideal soft materials for interacting with and manipulating biological structures.
However, the material properties of coacervates are not always constant over time (Fig. 2). While most (synthetic) complex coacervates made from oppositely charged polymers show no signs of aging, many protein-based coacervates have a viscosity that increases with age over the course of hours to days, while their elastic modulus varies weakly, likely due to a gradual increase in interactions between the proteins in the coacervate [8]. This time-dependent rheological behaviour is typical for glass-like materials, suggesting that protein-based coacervates are soft glassy materials that can be easily fluidized by changing composition, pH or temperature.
 |
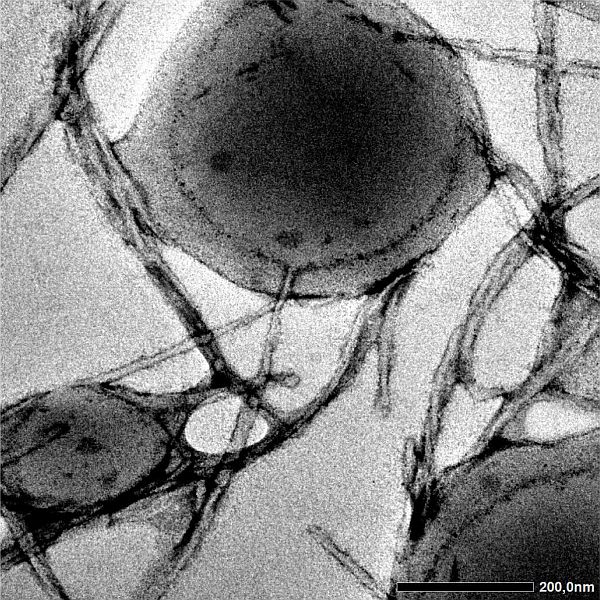
Fig. 2: Coacervate droplets are more than simple liquids: (a) aging of Maxwell fluids turns them into glassy solids. (b) metastable coacervate droplets can form amyloid-like fibres which primarily nucleate at the droplet interface. (c) Shear forces can accelerate the transformation of coacervate droplets into fibres. |
In some circumstances, the material properties of coacervates can change more quickly and drastically. It has been shown that the liquid condensed state of a coacervate is a metastable state for some disordered proteins. Eventually, these proteins will form highly structured amyloid-like fibres, but the kinetics of this process is usually slow because of a high energy barrier. Interestingly, the liquid coacervate itself can significantly accelerate the formation of fibres: heterogeneous nucleation at the interface of coacervates lowers the nucleation barrier for both fibres of proteins that make up the original coacervate and fibres of guest proteins that were later taken up by the coacervates [9]. Shear forces can also dramatically enhance the transformation of liquid coacervates into fibrous solids, with complete transformation occurring in seconds [10]. In this case, alignment of disordered proteins in shear flow facilitates their assembly into filaments.
Finally, the interplay between fibres and coacervates creates a rich field of soft matter that is largely unexplored. Coacervate wetting of large, flexible fibres can lead to bundling governed by an elastocapillary length, which is of the order of 1-100 nm for typical protein-based coacervates and filaments. Coacervates that are embedded in elastic networks formed by cytoskeletal filaments exhibit elastic ripening, migration and Plateau-Rayleigh instability. And short filaments inside coacervates can tune droplet shapes from spheres to ellipsoids and tactoids.
Sticky coacervates
The dynamic, phase-separated nature of coacervates, combined with their unique soft material properties, adhesion and ability to undergo rapid solidification, also make coacervates attractive for use in materials science. It comes as no surprise that several organisms have adopted coacervation to produce glues, biological fibres and composite materials. Protein coacervates are involved, for example, in the fabrication of spider silk, velvet worm slime, sandcastle worm cement, mussel byssus, and the squid beak [11].
In one of these examples, the sandcastle worm cement, phase separation and coacervate wetting helps sandcastle worms to build tubes from grains of sand that it glues together under difficult circumstances: the glue must be liquid when secreted, but hold in salty water without being washed away. To achieve this, the adhesive components are packaged at low pH in secretory granules. Upon secretion into (sea)water, the pH change causes rupture of the granules and triggers the complexation between oppositely charged adhesive components into a complex coacervate-like material that adheres to the wet mineral surfaces. Within tens of seconds, the glue has cured sufficiently to keep grains in place due a combination of glassy aging, magnesium/calcium exchange and enzymatic oxidation of tyrosine residues into dopa.
Another example is given by velvet worms. These creatures resemble Spider Man: they shoot lines of sticky liquid that can harden into solid threads upon impact. But unlike Spider Man, velvet worms use their threads to trap prey. The velvet worm’s slime is a sticky liquid mostly composed of disordered, highly charged proteins, with a small fraction of lipids. Upon ejection, the slime quickly hardens due to a combination of water evaporation, shear-induced structural rearrangements, lipid coating of fibres and disulfide bond formation. Interestingly, the fibres can be redissolved in water and recycled, suggesting that the proteins did not undergo irreversible aggregation, as in the case of amyloids.
The key advantage of coacervate-based adhesives and materials is their ability to work in challenging environments. Traditional glues often fail when surfaces are damp or contaminated, but coacervates can stick in such settings. Further chemical or physical aging of the coacervates then locks them in place as they harden. This not only makes them attractive for marine organisms and velvet worms, but also for applications in wound healing and for application of medical implants and devices.
Perspectives: coacervate therapeutics and coacervate cosmetics?
Apart from applications in biomaterials, smaller-scale coacervate droplets also hold great potential in controlled delivery due to their unique physicochemical properties. Coacervates can interact with lipid membranes in diverse ways, and multiple studies have reported delivery of micron-sized coacervate droplets into living cells. Wrapping of coacervates results in spontaneous uptake as membrane-enveloped structures, but lipid partitioning into the coacervates could result in disintegration of the membrane shell and release of the coacervate. This may open the way for direct delivery of RNA, proteins, and even complexes and capsids. Alternatively, in cosmetics and personal care products, coacervates could offer a mechanism for controlled delivery system of active ingredients by adhesion and permeation.
Coacervates represent a unique class of soft materials: they combine mechanical softness with phase separation, interfaces, capillarity and metastability, which offers many possibilities for interacting with and manipulating biological systems. Fundamentally, these properties all stem from the weak, multivalent interactions between molecules inside the coacervates, of a few kBT every few nm. By learning how to design synthetic molecules with these features that phase separate into materials with the right viscosity, (meta)stability, and stickiness, we will be able to mimic the remarkable features of nature into new types of smart materials, and - one day - even build synthetic life.
About the Author

Evan Spruijt is group leader of Coacervates and Soft Interfaces at Radboud University Nijmegen, the Netherlands. His research focuses on the physical chemistry of biomolecular condensates and coacervate protocells, and his group investigates the roles of coacervates in the origin of life and in cellular organization and disease.
References
- S. F. Banani, H. O. Lee, A. A. Hyman and M. K. Rosen, Nat Rev Mol Cell Biol 18, 285 (2017) [Google Scholar]
- J. Söding, D. Zwicker, S. Sohrabi-Jahromi, M. Boehning and J. Kirschbaum, Trends in Cell Biology 30, 4 (2020) [Google Scholar]
- R. V. Pappu, S. R. Cohen, F. Dar, M. Farag and M. Kar, Chem. Rev. 123, 8945 (2023) [Google Scholar]
- E. Spruijt, M. A. Cohen Stuart and J. van der Gucht, Macromolecules 46, 1633 (2013) [Google Scholar]
- K. L. Weirich, K. Dasbiswas, T. A. Witten, S. Vaikuntanathan and M. L. Gardel, Proc. Natl. Acad. Sci. U.S.A. 116, 11125 (2019). [Google Scholar]
- Y. Wang et al., Nature 634, 1204 (2024) [Google Scholar]
- A. R. Strom et al., Cell 187, 5282.e20 (2024) [Google Scholar]
- L. Jawerth et al., Science 370, 1317 (2020) [Google Scholar]
- B. S. Visser, W. P. Lipiński and E. Spruijt, Nat Rev Chem 8, 686 (2024) [Google Scholar]
- Y. Shen et al., Nat. Nanotechnol. 15, 841 (2020) [Google Scholar]
- M. J. Harrington, R. Mezzenga, and A. Miserez, Nat Rev Bioeng 2, 260 (2023) [Google Scholar]
All Figures
 |
Fig. 1: Coacervates as phase-separated droplets in biology: (a) A mammalian cell with various types of membraneless organelles, (b) free exchange of proteins and small molecules in one such coacervate-based compartment due to a lack of membrane, (c) example of liquid nature of phase-separated droplets of small heat shock protein B2 (HspB2) in the nucleus of HEK293 cells, (d) synthetic coacervate droplets made from short peptide derivatives, (e) upon fusion and settling, coacervates can form macroscopic dense liquid phases. The position of the interface is highlighted with an arrow. |
| In the text | |
 |
Fig. 2: Coacervate droplets are more than simple liquids: (a) aging of Maxwell fluids turns them into glassy solids. (b) metastable coacervate droplets can form amyloid-like fibres which primarily nucleate at the droplet interface. (c) Shear forces can accelerate the transformation of coacervate droplets into fibres. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


