Thermodiffusion in weightlessness (Vol. 46 No. 1)
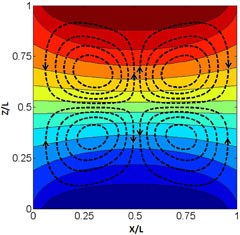
Credit: Y. Gaponenko et al.
Thermodiffusion, also called the Soret effect, is a mechanism by which an imposed temperature difference establishes a concentration difference within a mixture. Two recent studies provide a better understanding of such effects. They build on recent experimental results from the IVIDIL—Influence Vibration on Diffusion in Liquids—research project performed on the International Space Station under microgravity to avoid motion in the liquids.
In the first study, using a mathematical model the authors set out to identify how vibrations applied to a binary liquid mixture change the temperature and concentration fields over a long time scale. Their findings—if extended to ternary mixtures—have implications for models used to evaluate the economic value of oil reservoirs.
The second paper use numerical models to study the establishment of the concentration field near the critical region, where diffusion strongly diminishes. Surprisingly, the authors demonstrate that the component separation through the Soret effect is saturated and not infinite, and is reached surprisingly rapidly. The findings of this study may help determine whether the Soret effect could lead to a very large accumulation of sulfur dioxide and hydrogen sulphide capable of creating a leak in the cap-rock of a reservoir, during the process of capturing CO2 and reinjecting it in supercritical state in such a reservoir.
Y. Gaponenko, A. Mialdun and V. Shevtsova, “Experimental and numerical analysis of mass transfer in a binary mixture with Soret effect in the presence of weak convection”, Eur. Phys. J. E 37, 90, (2014)
[Abstract]
J.C. Legros, Yu. Gaponenko, T. Lyubimova and V. Shevtsova, “Soret separation in a binary liquid mixture near its critical temperature”, Eur. Phys. J. E 37, 89 (2014)
[Abstract]







